Abstract
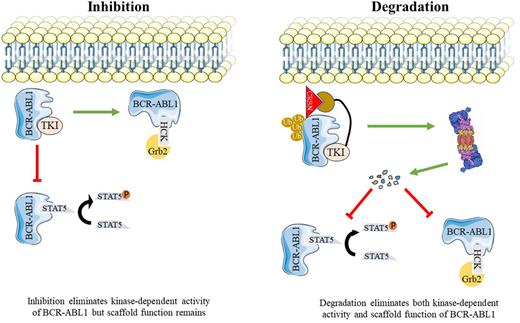
Chronic Myeloid Leukemia (CML) is a myeloproliferative neoplasm caused by BCR-ABL1, a constitutively active tyrosine kinase resulting from the t(9;22)(q34;q11.2). Tyrosine kinase inhibitors (TKIs) such as imatinib that competitively target the orthosteric site (ATP binding pocket) of BCR-ABL1 have markedly improved survival of patients with chronic phase (CP)-CML. Recently, the first clinical BCR-ABL1 allosteric inhibitor, asciminib was approved for the treatment of patients who failed at least two prior TKIs. Despite these advances, significant clinical challenges remain. Only one-quarter of patients achieve treatment free remission (TFR) and approximately 10% progress to blast phase (BP)-CML. There is compelling evidence that kinase-independent functions of BCR-ABL1 contribute to leukemogenesis, perhaps reflecting a scaffold function of BCR-ABL1 that promotes activation of certain signaling pathways. Disruption of these functions will require elimination of BCR-ABL1 protein. PROTACs (Proteolysis-Targeting Chimeras) are heterobifunctional molecules that can facilitate target protein degradation by the proteasomal machinery. BCR-ABL1 PROTACs reported so far are active in the micromolar range and their ability to degrade BCR-ABL1 compound mutants have yet-to-be evaluated. No clinical BCR-ABL1 PROTAC has been reported.
As a first step toward developing potent PROTACs that selectively target native and mutant BCR-ABL1, we synthesized a library of 52 asciminib-based PROTACs composed of asciminib as a target warhead connected via a linker to a E3 ubiquitin ligase ligand. Our platform allows for synthesis and screening of hundreds of compounds with different linkers and various E3 ligase recruiters. To facilitate medium throughput screening and perform initial validation of candidates, we developed a Nanoluc protein complementation assay. In this assay, BCR-ABL1 is N-terminally tagged with a small Nanoluc fragment (HiBiT) that binds with high affinity to the larger Nanoluc fragment (LgBiT). The HiBiT-LgBiT complex functions as active luciferase, allowing to measure BCR-ABL1 levels in intact cells. From 52 PROTACs, we identified LPA81 as the most active candidate. LPA81 degrades more than 90% of BCR-ABL1 within 12 hours at 0.5 µM. In addition, LPA81 showed high degradation potency against BCR-ABL1 single mutants at concentration as low as 0.5 µM which is nearly 2-fold higher than that of published PROTACs. Importantly, LPA81's activity extended to compound mutant BCR-ABL1. Next, immunoblotting was performed to further assess the kinetics and potency of LPA81, showing that LPA81 degrades BCR-ABL1 in a dose- and time-dependent fashion. Interestingly, LPA81 showed degradation potency at concentration as low as 10nM and as early as 4 hours. The maximal level of degradation (Dmax) of LPA81 was 98% with a DC50=0.01 μmol/L within 24hours. Pre-treatment of cells with the proteasome inhibitor, MG132, but not the lysosomal inhibitor, chloroquine, reversed degradation of BCR-ABL1, confirming that BCR-ABL1 degradation by LPA81 occurs via 26s-proteasome. To assess the target selectivity, we applied cytotoxicity assay on human CML cell lines (K562 and LAMA-84) as well as BaF3 cells expressing either native or mutant BCR-ABL1 (T315I, E255V, Y253H/E255V, and H396R/T315I). LPA81 induced cytotoxicity in all tested cell models and IC50 values were measured less than 1µM, however, no toxicity was observed against BaF3 cells up to 10µM, confirming selectivity.
To directly evaluate the binding affinity of LPA81 to the myristoyl pocket of ABL1, the NanoBRET competitive binding assay was performed using our newly developed cell permeant asciminib-based fluorescent tracer as an acceptor and Nanoluc fusion ABL1 as a donor. By comparison, EC50 values for asciminib and LPA81 were calculated at 10.5nM and 300nM, respectively, reflecting either weaker LPA81 binding affinity compared to asciminib or reduced cellular uptake.
Collectively, our data suggest that LPA81 is an exceptionally potent first-in-class PROTAC capable of killing CML cells through degrading native and mutant BCR-ABL1 via ubiquitin-proteasomal system. Cellular uptake experiments and efficacy studies in primary cells, including comparisons with asciminib and the LPA81-related negative control which has equivalent cell permeability to LPA81 but lacks degradation activity, are underway and will be presented.
Disclosures
Deininger:Pfizer Inc: Consultancy, Honoraria, Research Funding; Bristol Myers Squibb: Consultancy, Honoraria, Research Funding; Galena Biopharma: Consultancy, Honoraria; Incyte: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Blueprint Medicines Corporation: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal